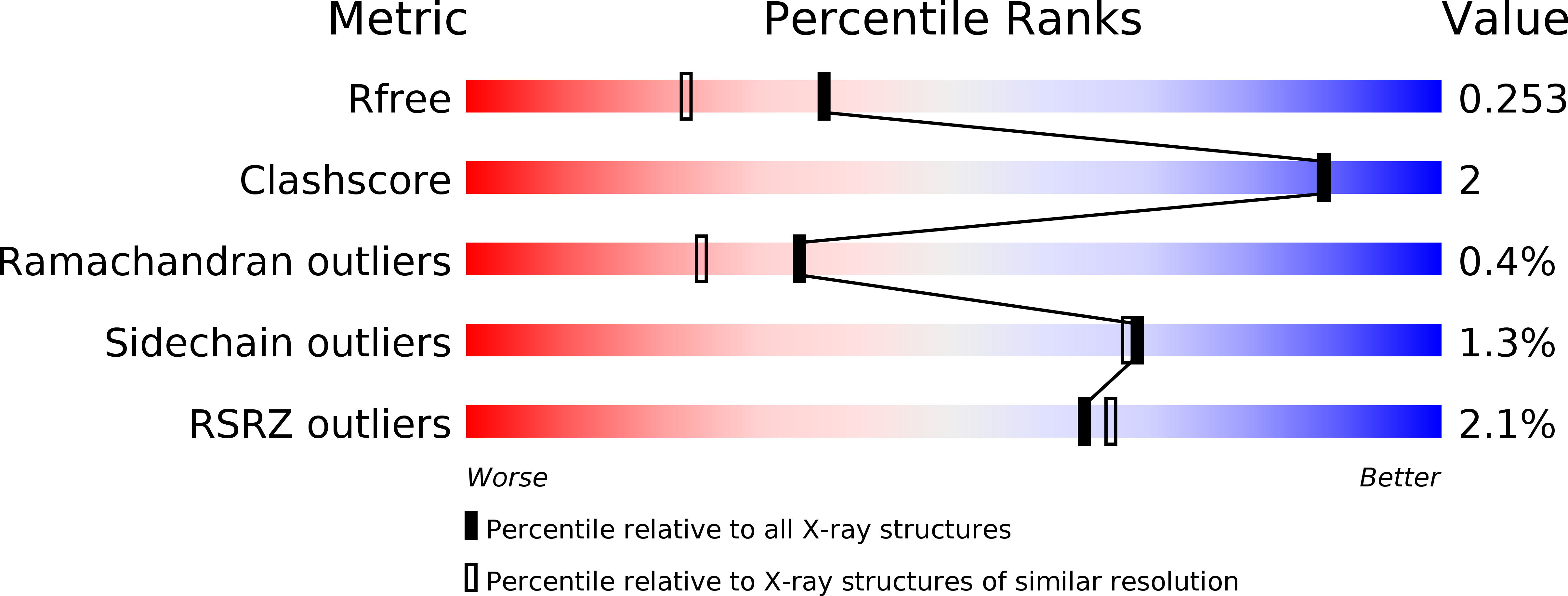
Nucleotide Binding, Evolutionary Insights, and Interaction Partners of the Pseudokinase Unc-51-like Kinase 4.
Preuss, F., Chatterjee, D., Mathea, S., Shrestha, S., St-Germain, J., Saha, M., Kannan, N., Raught, B., Rottapel, R., Knapp, S.(2020) Structure 28: 1184
- PubMed: 32814032
- DOI: https://doi.org/10.1016/j.str.2020.07.016
- Primary Citation of Related Structures:
6TSZ - PubMed Abstract:
Unc-51-like kinase 4 (ULK4) is a pseudokinase that has been linked to the development of several diseases. Even though sequence motifs required for ATP binding in kinases are lacking, ULK4 still tightly binds ATP and the presence of the co-factor is required for structural stability of ULK4. Here, we present a high-resolution structure of a ULK4-ATPγS complex revealing a highly unusual ATP binding mode in which the lack of the canonical VAIK motif lysine is compensated by K39, located N-terminal to αC. Evolutionary analysis suggests that degradation of active site motifs in metazoan ULK4 has co-occurred with an ULK4-specific activation loop, which stabilizes the C helix. In addition, cellular interaction studies using BioID and biochemical validation data revealed high confidence interactors of the pseudokinase and armadillo repeat domains. Many of the identified ULK4 interaction partners were centrosomal and tubulin-associated proteins and several active kinases suggesting interesting regulatory roles for ULK4.
Organizational Affiliation:
Institute for Pharmaceutical Chemistry, Johann Wolfgang Goethe-University, Max-von-Laue-Str. 9, 60438 Frankfurt am Main, Germany; Buchmann Institute for Molecular Life Sciences, Structural Genomics Consortium, Johann Wolfgang Goethe-University, Max-von-Laue-Str. 15, 60438 Frankfurt am Main, Germany.