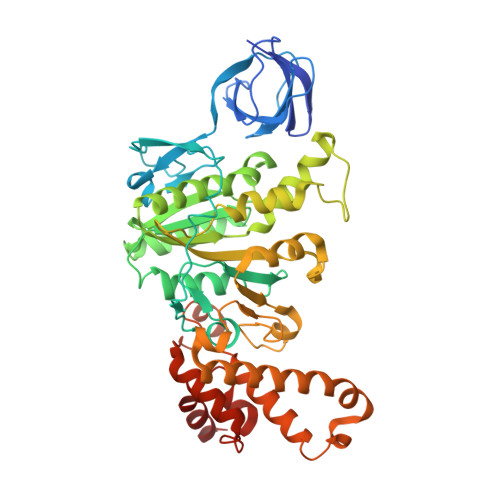
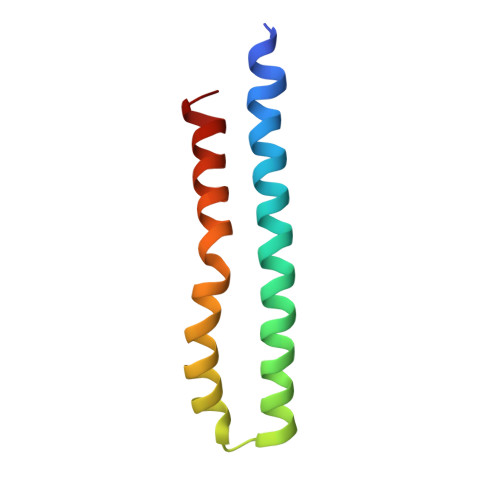
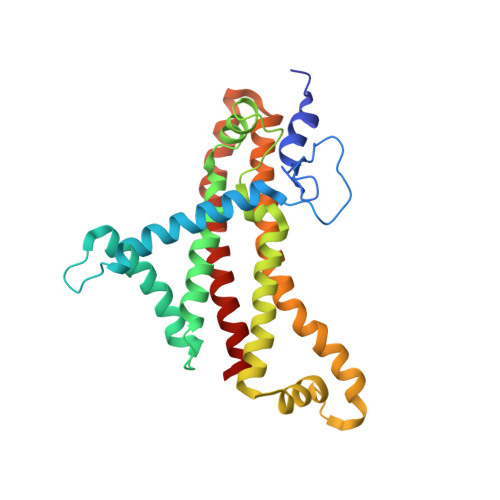
Cryo-EM structures provide insight into how E. coli F1FoATP synthase accommodates symmetry mismatch.
Sobti, M., Walshe, J.L., Wu, D., Ishmukhametov, R., Zeng, Y.C., Robinson, C.V., Berry, R.M., Stewart, A.G.(2020) Nat Commun 11: 2615-2615
- PubMed: 32457314
- DOI: https://doi.org/10.1038/s41467-020-16387-2
- Primary Citation of Related Structures:
6OQR, 6OQS, 6OQT, 6OQU, 6OQV, 6OQW, 6PQV, 6VWK, 6WNQ, 6WNR - PubMed Abstract:
F 1 F o ATP synthase functions as a biological rotary generator that makes a major contribution to cellular energy production. It comprises two molecular motors coupled together by a central and a peripheral stalk. Proton flow through the F o motor generates rotation of the central stalk, inducing conformational changes in the F 1 motor that catalyzes ATP production. Here we present nine cryo-EM structures of E. coli ATP synthase to 3.1-3.4 Å resolution, in four discrete rotational sub-states, which provide a comprehensive structural model for this widely studied bacterial molecular machine. We observe torsional flexing of the entire complex and a rotational sub-step of F o associated with long-range conformational changes that indicates how this flexibility accommodates the mismatch between the 3- and 10-fold symmetries of the F 1 and F o motors. We also identify density likely corresponding to lipid molecules that may contribute to the rotor/stator interaction within the F o motor.
Organizational Affiliation:
Molecular, Structural and Computational Biology Division, The Victor Chang Cardiac Research Institute, Darlinghurst, NSW, 2010, Australia.