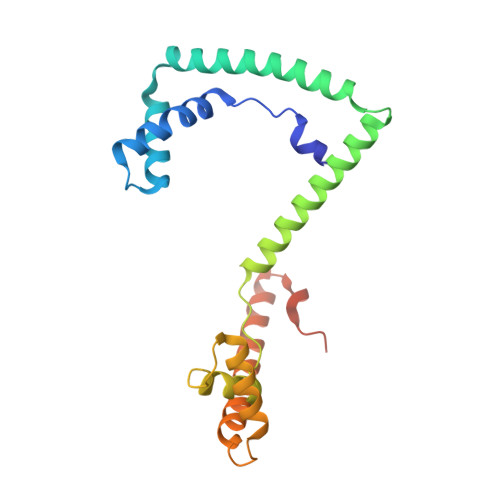
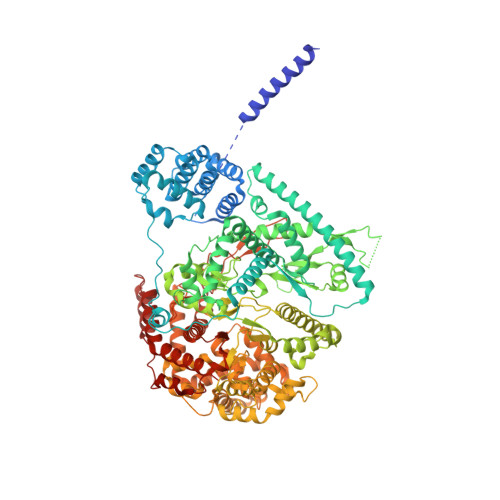
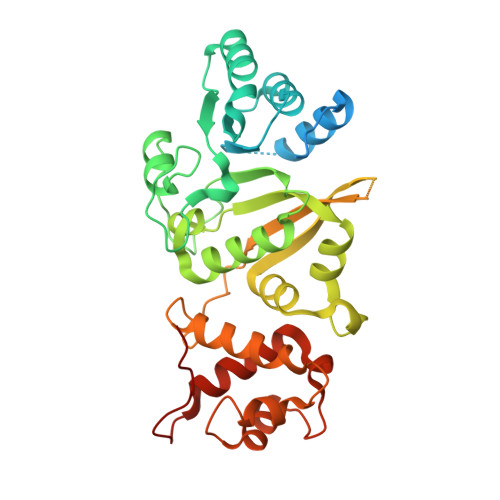
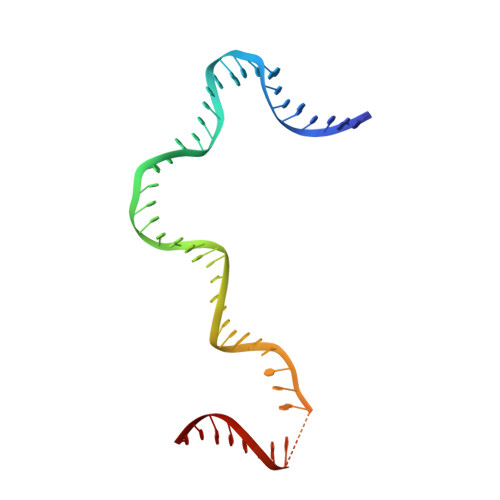
Structural Basis of Mitochondrial Transcription Initiation.
Hillen, H.S., Morozov, Y.I., Sarfallah, A., Temiakov, D., Cramer, P.(2017) Cell 171: 1072-1081.e10
- PubMed: 29149603
- DOI: https://doi.org/10.1016/j.cell.2017.10.036
- Primary Citation of Related Structures:
6ERO, 6ERP, 6ERQ - PubMed Abstract:
Transcription in human mitochondria is driven by a single-subunit, factor-dependent RNA polymerase (mtRNAP). Despite its critical role in both expression and replication of the mitochondrial genome, transcription initiation by mtRNAP remains poorly understood. Here, we report crystal structures of human mitochondrial transcription initiation complexes assembled on both light and heavy strand promoters. The structures reveal how transcription factors TFAM and TFB2M assist mtRNAP to achieve promoter-dependent initiation. TFAM tethers the N-terminal region of mtRNAP to recruit the polymerase to the promoter whereas TFB2M induces structural changes in mtRNAP to enable promoter opening and trapping of the DNA non-template strand. Structural comparisons demonstrate that the initiation mechanism in mitochondria is distinct from that in the well-studied nuclear, bacterial, or bacteriophage transcription systems but that similarities are found on the topological and conceptual level. These results provide a framework for studying the regulation of gene expression and DNA replication in mitochondria.
Organizational Affiliation:
Department of Molecular Biology, Max Planck Institute for Biophysical Chemistry, Am Fassberg 11, 37077 Göttingen, Germany.