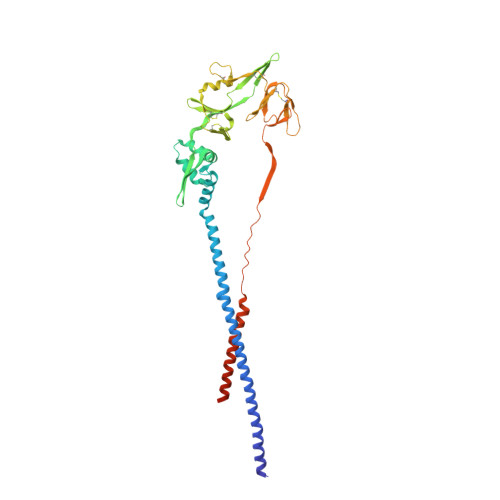
Engineering, Structure and Immunogenicity of the Human Metapneumovirus F Protein in the Postfusion Conformation.
Mas, V., Rodriguez, L., Olmedillas, E., Cano, O., Palomo, C., Terron, M.C., Luque, D., Melero, J.A., McLellan, J.S.(2016) PLoS Pathog 12: e1005859-e1005859
- PubMed: 27611367
- DOI: https://doi.org/10.1371/journal.ppat.1005859
- Primary Citation of Related Structures:
5L1X - PubMed Abstract:
Human metapneumovirus (hMPV) is a paramyxovirus that is a common cause of bronchiolitis and pneumonia in children less than five years of age. The hMPV fusion (F) glycoprotein is the primary target of neutralizing antibodies and is thus a critical vaccine antigen. To facilitate structure-based vaccine design, we stabilized the ectodomain of the hMPV F protein in the postfusion conformation and determined its structure to a resolution of 3.3 Å by X-ray crystallography. The structure resembles an elongated cone and is very similar to the postfusion F protein from the related human respiratory syncytial virus (hRSV). In contrast, significant differences were apparent with the postfusion F proteins from other paramyxoviruses, such as human parainfluenza type 3 (hPIV3) and Newcastle disease virus (NDV). The high similarity of hMPV and hRSV postfusion F in two antigenic sites targeted by neutralizing antibodies prompted us to test for antibody cross-reactivity. The widely used monoclonal antibody 101F, which binds to antigenic site IV of hRSV F, was found to cross-react with hMPV postfusion F and neutralize both hRSV and hMPV. Despite the cross-reactivity of 101F and the reported cross-reactivity of two other antibodies, 54G10 and MPE8, we found no detectable cross-reactivity in the polyclonal antibody responses raised in mice against the postfusion forms of either hMPV or hRSV F. The postfusion-stabilized hMPV F protein did, however, elicit high titers of hMPV-neutralizing activity, suggesting that it could serve as an effective subunit vaccine. Structural insights from these studies should be useful for designing novel immunogens able to induce wider cross-reactive antibody responses.
Organizational Affiliation:
Unidad de Biología Viral, Centro Nacional de Microbiología and CIBER de Enfermedades Respiratorias, Instituto de Salud Carlos III, Majadahonda, Madrid, Spain.